Abstract
Despite the use of of intensive chemotherapy, and allogeneic stem cell transplant (HSCT) in selected cases, children with acute myeloid leukemia (AML) have 5-year overall survival (OS) rates of ~65%. Apart from early clinical response, cytogenetic and molecular aberrations are the most reliable prognostic factors for survival. Identifying prognostic subgroups to stratify patients for treatment in future trials is clinically important. The study of rare AML subtypes can be facilitated by international collaborations. AML with t(16;21) rearrangements includes 2 different translocations: t(16;21)(p11;q22) resulting in the FUS-ERG fusion, and t(16;21)(q24;q22) resulting in the RUNX1-CBFA2T3 fusion.
To study outcomes and associated prognostic factors, clinical data from children with t(16;21) AML were collected from 14 study groups participating in the international Berlin-Frankfurt-Munster (I-BFM) network. Patients (age 0-18 years) diagnosed from January 1, 1995, to January 1, 2016, were included. The AML-BFM study group provided data from 1326 unselected patients diagnosed between 1997 and 2013 as a reference cohort. All karyotypes were centrally reviewed by 2 independent cytogeneticists according to the International System for Human Cytogenetic Nomenclature (ISCN 2005). Patients with inconclusive karyotypes were screened by reverse-transcription PCR. Chi-squared and Fisher exact tests were used to compare clinical characteristics. OS and event free survival (EFS) analysis was performed by the Kaplan-Meier method. Cumulative incidence of relapse (CIR) was calculated according to Kalbfleisch and Prentice. The Cox proportional hazards model was used for multivariate analysis, considering as covariables age, white blood cell (WBC) count at diagnosis, cytogenetic risk group and allogeneic hematopoietic stem cell transplantation (HSCT) as time-dependent variable.
There were 54 patients with t(16;21): 32 with FUS-ERG and 22 with RUNX1-CBFA2T3 . There were no significant differences in sex and age between patients in the FUS-ERG- or RUNX1-CBFA2T3- groups and the reference cohort. Median WBC at diagnosis was significantly lower for patients with RUNX1-CBFA2T3 (12.5×109/L, range 0.01-185×109/L) than for the reference cohort (19.4×109/L, range 0.01-1900×109/L, P=0.030). Patients with FUS-ERG had no predominant French-American-British (FAB) type, whereas 76.4% of those with RUNX1-CBFA2T3 had a granulocytic FAB type (M1, M2) compared with 42.1% in the reference cohort (P=0.004).
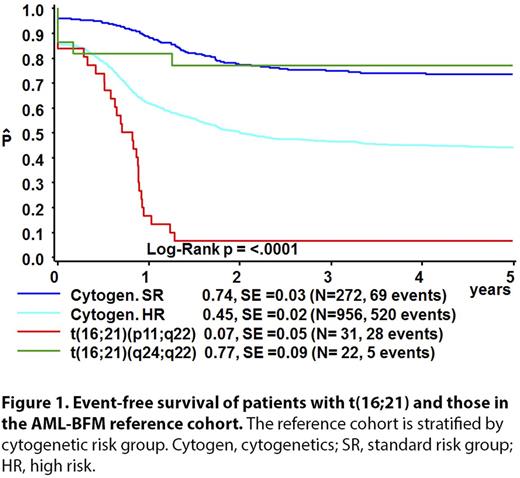
The 4-year EFS, OS, and CIR for the reference cohort were 51% [standard error (SE)=1%], 68% (SE=1%), and 32% (SE=1%), respectively. Median follow up for survivors in the t(16;21) cohort was 1.6 years for those with FUS-ERG and 5.0 years for RUNX1-CBFA2T3 . Patients with FUS-ERG had a 4-year EFS of 7% (SE=5%, P <0.0001), 4-year OS of 21% (SE=8%, P <0.0001), and CIR of 74% (SE=8%, P <0.0001). Patients with RUNX1-CBFA2T3 had a 4-year EFS of 77% (SE=9%, P=0.06), OS of 81% (SE=8%, P=0.34) and a CIR of 0%. When patients in the reference cohort were classified in SR patients comprising (inv(16)/t(8;21), and HR patients comprising other cytogenetic subtypes excluding t(16;21), EFS rates were 74% (SE=3%) and 45% (SE=2%), respectively (Fig. 1). Hence, patients with RUNX1-CBFA2T3 had a similar outcomes as SR patients (Figure 1). In multivariate analysis, presence of FUS-ERG and RUNX1-CBFA2T3 were independent risk factors for EFS, with hazard ratios of 1.93 (P <0.0001) and 0.325 (P=0.025), respectively. HSCT was performed in 14 (45.2%) patients with FUS-ERG in first complete remission (CR1). The 4-year EFS for transplanted patients was 15% (SE=15%) compared with 0% (SE=13%) for patients receiving chemotherapy only (p=NS).
We identified 2 clinically relevant, distinct subtypes of pediatric AML patients with different t(16;21) rearrangements: FUS-ERG had poor outcomes, whereas RUNX1-CBFA2T3 had favorable outcomes. Patients with RUNX1-CBFA2T3 -rearranged AML might benefit from inclusion in the SR group and receiving less intensive treatment, whereas those with FUS-ERG rearranged AML should be stratified in the HR group. In this study, only patients with FUS-ERG receiving HSCT in CR1 did not relapse. Most current pediatric AML trials consider children with a 5-year OS below 25%-30% for HSCT in CR1.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal